Visualizing Equilibrium with Cobalt Complexes
———————————————————————————————————————————
Background
———————————————————————————————————————————
Many students have struggled with introductory chemistry during their freshman years in college (1). It has been shown that college-level chemistry does not accommodate students from various high school chemistry backgrounds. The typical blackboard lectures are limited to calculations and freeze-framed chalk drawings, which do not capture the truly dynamic nature of chemical phenomena, and cater to students who already have a strong arithmetic background. This poses a problem for students who do not possess sufficient arithmetic skills to succeed in introductory chemistry, making this course less accessible to those who demonstrate interest in this subject (2, 3).
I spent four years of my time at Brown University developing the experiment, iterating it with feedback from students for each semester, and repurposing it for online learning during the pandemic. I continued to be involved in this project after graduation, specifically with literature review and feedback. Here are the publications that I contributed on for this project, which you can find below:

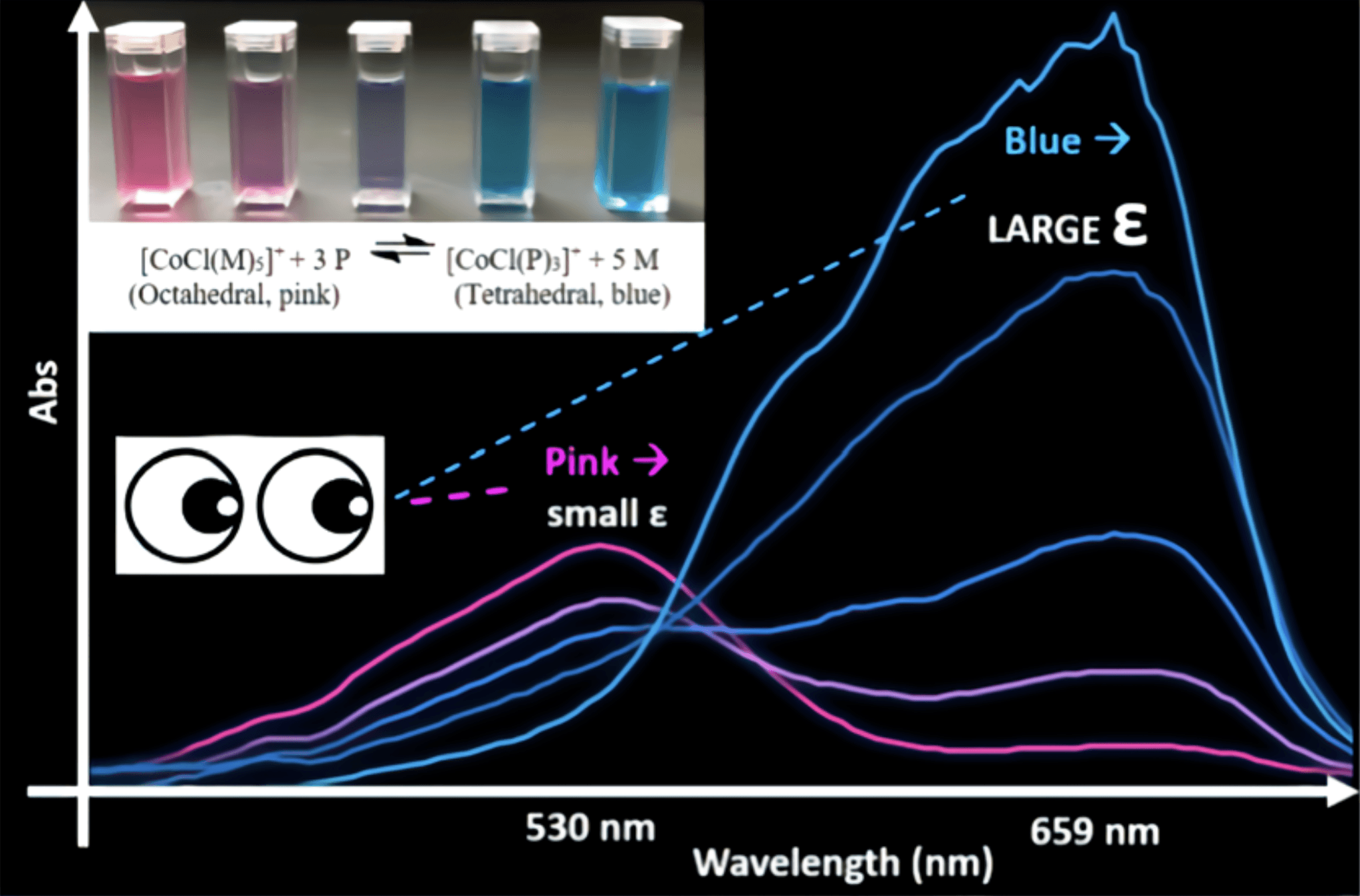
Exploring Chemical Equilibrium for Alcohol-Based Cobalt Complexation through Visualization of Color Change and UV–vis Spectroscopy
January 2020
An in-depth peer-reviewed publication that outlines the entire process of designing an experiment that visualizes equilibrium. Includes methods, results, and student feedback. Published as first-author.
Read article

Strategies, Practice and Lessons Learned from Remote Teaching of the General Chemistry Laboratory Course at Brown University
August 2020
A review of how I and my research group digitized three laboratory experiments during the pandemic for online learning. We share our experiences, strategies, and lessons learned from teaching chemistry lab remotely. Published as co-author.
Read article

Innovative Approaches for Student-Led Creation of Animated and Interactive Videos in an Undergraduate Introductory Chemistry Course
July 2025
This paper details the student-led approach to video production and highlights how peer-developed content can enhance engagement and accessibility. I contributed to the three interactive videos mentioned in the “Discussion.”
Read article
I am beyond thankful for the support and guidance of my research and thesis mentor, Dr. Li-Qiong Wang, Ph.D. For the four years that I have been involved in her lab, Dr. Wang encouraged me to go above my level of caliber and allowed me to propose a research project for CHEM0330 during my first year in undergraduate. I’m grateful for her patience and trust in my curiosity to learn about scientific research, from literature review to publication, and the time Dr. Wang has invested in my growth. I am also thankful for Tiffany and Len for helping me edit the manuscript and for their contribution to these projects. Additional thanks go to Giovanna Roz Gastaldi and the team at Brown Media Services to help us animate and bring chemistry to life through technology.
Acknowledgements
———————————————————————————————————————————
1) Frey, R. F.; Cahill, M. J.; McDaniel, M. A. Students’ Concept Building Approaches: A Novel Predictor of Success in Chemistry Courses. J. Chem. Educ. 2017, 94 (9), 1185-1194. https://doi.org/10.1021/acs.jchemed.7b00059.
2) Nicole M. Grove; Mary B. Nakhleh. Factors That Affect Students’ Positive and Negative Perceptions of Chemistry, 2008. http://oasys2.confex.com/acs/235nm/techprogram/P1163770.HTM.
3) Powell, C. B.; Simpson, J.; Williamson, V. M.; Dubrovskiy, A.; Walker, D. R.; Jang, B.; Shelton, G. R.; Mason, D. Impact of Arithmetic Automaticity on Students’ Success in Second-Semester General Chemistry. Chem. Educ. Res. Pract. 2020, 21 (4), 1028–1041. https://doi.org/10.1039/D0RP00006J.
Citations
———————————————————————————————————————————